
Pathology Cases LN review
The well-differentiated or "mature" plasma cell (so called Marshalko-type) shows the characteristic round eccentric nuclei with "clock face" chromatin without nucleoli and abundant dense basophilic cytoplasm with clear perinuclear hof corresponding to the Golgi zone.

Clockdependent chromatin topology modulates circadian transcription
Plasma cells (arrows) have eccentric nuclei characterized by a "clock face" appearance to the chromatin. The cytoplasm may range from basophilic to blue-gray and can contain vacuoles. Download Image Share Image Views: 10344 Downloads: 42 Size: 0.16 MB This image belongs to set: Plasma cells Download Set

Cold‐induced chromatin compaction and nuclear retention of clock mRNAs
Chromatin is heavily clumped, and dense masses of chromatin show the typical "spoke wheel" or "clock face" chromatin pattern. Nucleolus is not visible. The cytoplasm is abundant, always basophilic and usually deep blue. A well-defined large and colorless perinuclear hof is present in almost all PC and corresponds to the uncolored Golgi.

Transcriptional Architecture and Chromatin Landscape of the Core
The chromatin at clock-controlled genes undergoes rhythmic modifications mediated by the activities of histone modifiers and chromatin-remodeling proteins, thus facilitating rhythmic gene expression over the 24-hour cycle [29-31]. There is accumulating evidence showing that these proteins interact with core clock components to impose temporal.

Chromatin landscape and circadian dynamics Spatial and temporal
Abstract. Chromatin organization plays a crucial role in gene regulation by controlling the accessibility of DNA to transcription machinery. While significant progress has been made in understanding the regulatory role of clock proteins in circadian rhythms, how chromatin organization affects circadian rhythms remains poorly understood.
Pathology of Multiple Myeloma Pathology Made Simple
Definition Solitary lesions of clonal plasma cells that are cytologically, immunophenotypically, and genetically similar to plasma cell myeloma. Clinical Features Bone pain and cord compression due to vertebral lesions. Localization Dura-based (D.D. meningioma), intrasellar (D.D. pituitary adenoma), rarely intraparenchymal.

The plasma cell clockface or cartwheel nuclear pattern as seen in 2D
"Clock-face" chromatin pattern. Small dots symmetrically rim the nuclear membrane - like the numbers on a clock. Abundant cytoplasm. Nucleus-to-cytoplasm ratio ~1:2 Perinuclear hof (prominent Gogli apparatus). Pale perinuclear crescentic - may be up to the size of the nucleus in active plasma cells. Note:

Transcriptional activation and repression by circadian clockassociated
summary Multiple Myeloma is neoplastic proliferation of plasma cells that commonly results in multiple skeletal lesions, hypercalcemia, renal insufficiency, and anemia. Patients typically present at ages > 40 with localized bone pain or a pathologic fracture. Diagnosis is made with a bone marrow biopsy showing monoclonal plasma cells ≥10%.

Transcriptional Architecture and Chromatin Landscape of the Core
Clockface chromatin Plasma cells have distinctive features that are clearly seen in this electron micrograph: a prominent Golgi; well developed rough endoplasmic reticulum; and a nucleus with large clumps of heterochromatin at the margin of the nucleus (clock-face nucleus).. (clock-face nucleus). Compare these features with the high.

Hormone Pulses and Transcription Bursting Falconieri Visuals LLC
Pathophysiology refers to changes in bodily processes that result from disease. In the case of multiple myeloma (MM), which is a type of bone marrow cancer, the pathophysiology is complex. It can.

Schematic model depicting the dynamic chromatin changes at the core of
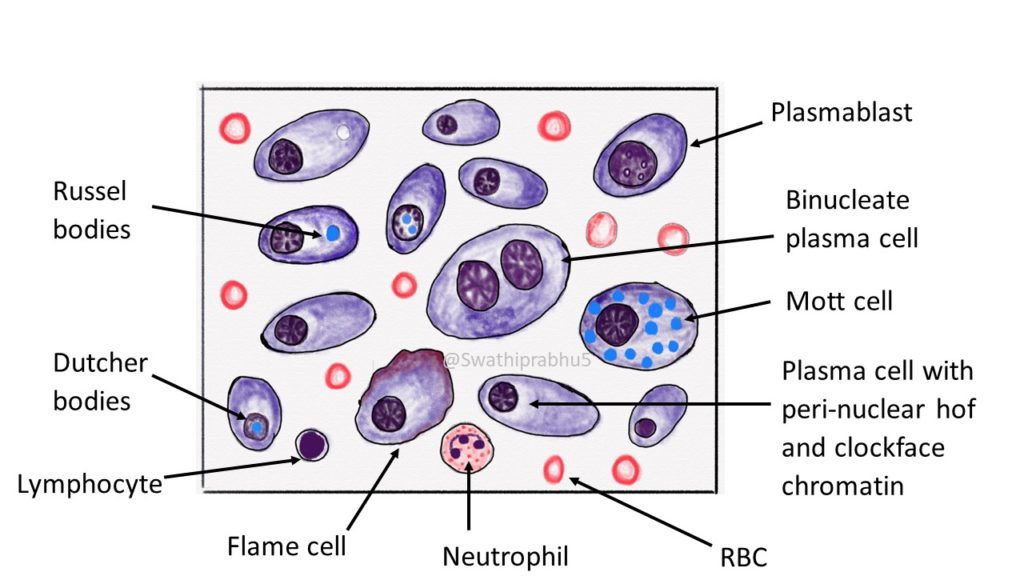
Eccentric nucleus with clock-face chromatin; Now 2/3 of cell is cytoplasm; Russell body: plasma cell that is filled with antibodies and the nucleus has been pushed out. Sign of really chronic inflammation Blood smear with immune cell s. Giant Cells. Syncytium of merged epithelioid histiocytes with multiple nuclei Multinucleated Langhans giant cell

Chromatin landscape and circadian dynamics Spatial and temporal
Plasma cells with prominent clock face chromatin Russell bodies Scattered immature plasma cells Scattered Mott cells with grape-like inclusions Board review style answer #3. D. Scattered immature plasma cells are more specific to a neoplastic process compared to binucleation, Russell bodies or mild plasmacytosis.

Chromatin Structure and Function as a Biological Clock during Aging
Mature plasma cells: oval with abundant basophilic cytoplasm, perinuclear hof, round eccentric nuclei, "clock face" chromatin and indiscernible nucleoli Immature plasma cells: higher nuclear / cytoplasmic ratio, more abundant cytoplasm and hof region compared to plasmablastic, more dispersed chromatin, often prominent nucleoli.

Chromatin landscape and circadian dynamics Spatial and temporal
Chromatin associated with the nuclear membrane gives the nuclei the appearance of a clock-face or cartwheel. The cytoplasm contains a prominent Golgi apparatus and endoplasmic reticulum. The precursors of plasma cells are plasmablasts which migrate to bone marrow after stimulation by cytokines from helper T cells in the germinal centers of.

The many faces of chromatin assembly factor 1 Trends in Plant Science
Abstract In the fully-developed megaloblast of vitamin B 12 or folate deficiency, unique alterations occur in the chromatin adherent to the nuclear membrane. This chromatin is often tenuously connected to or separated from other chromatin, and gives the nucleus a clockface appearance.

Plasmablastic lymphoma cells with a plasmacytoid appearence with a
Definition / general. Usually less than 1% of marrow cells; rare in infants. Often perivascular and in particle crush specimens. Indeterminate lifespan ranging from days to months. Produces and secretes antibodies. Plasmablast: precursor to plasma cell, produces more antibodies than B cells but less than mature plasma cells.